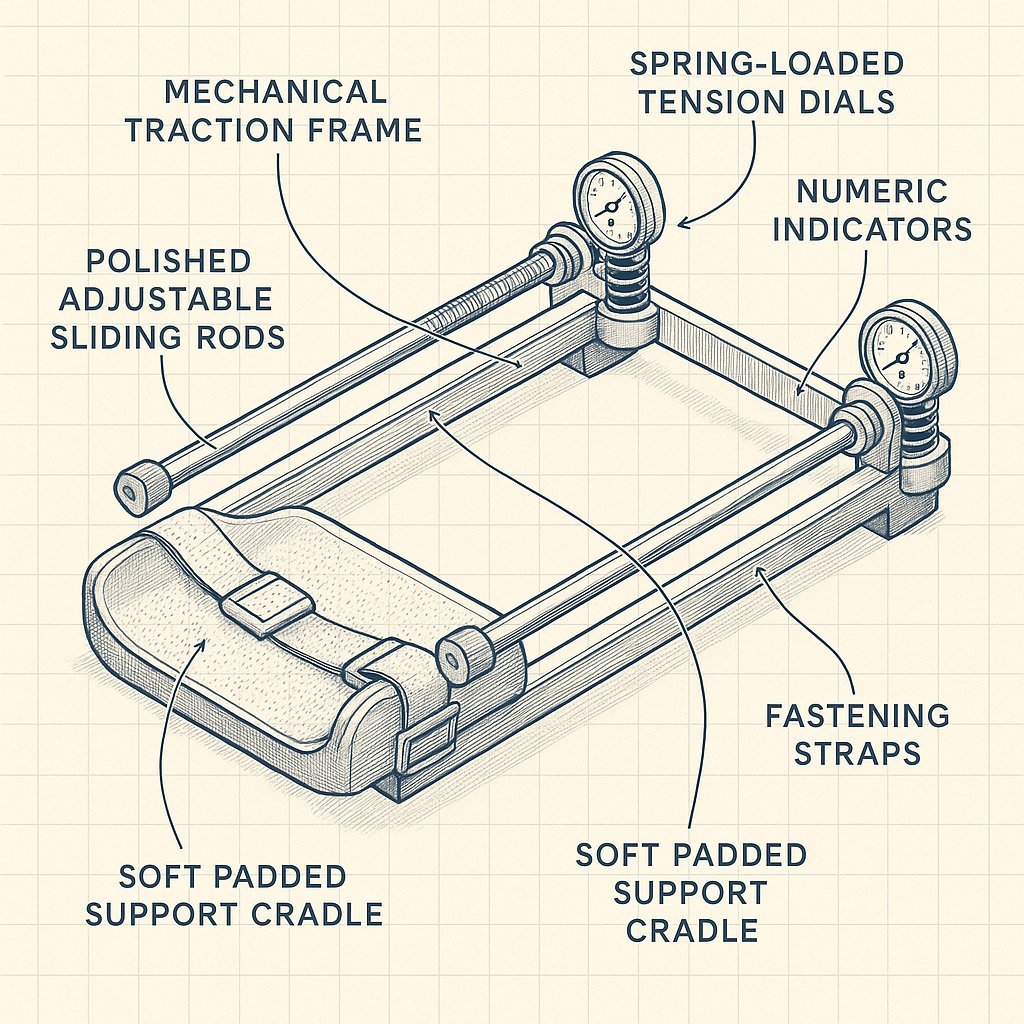
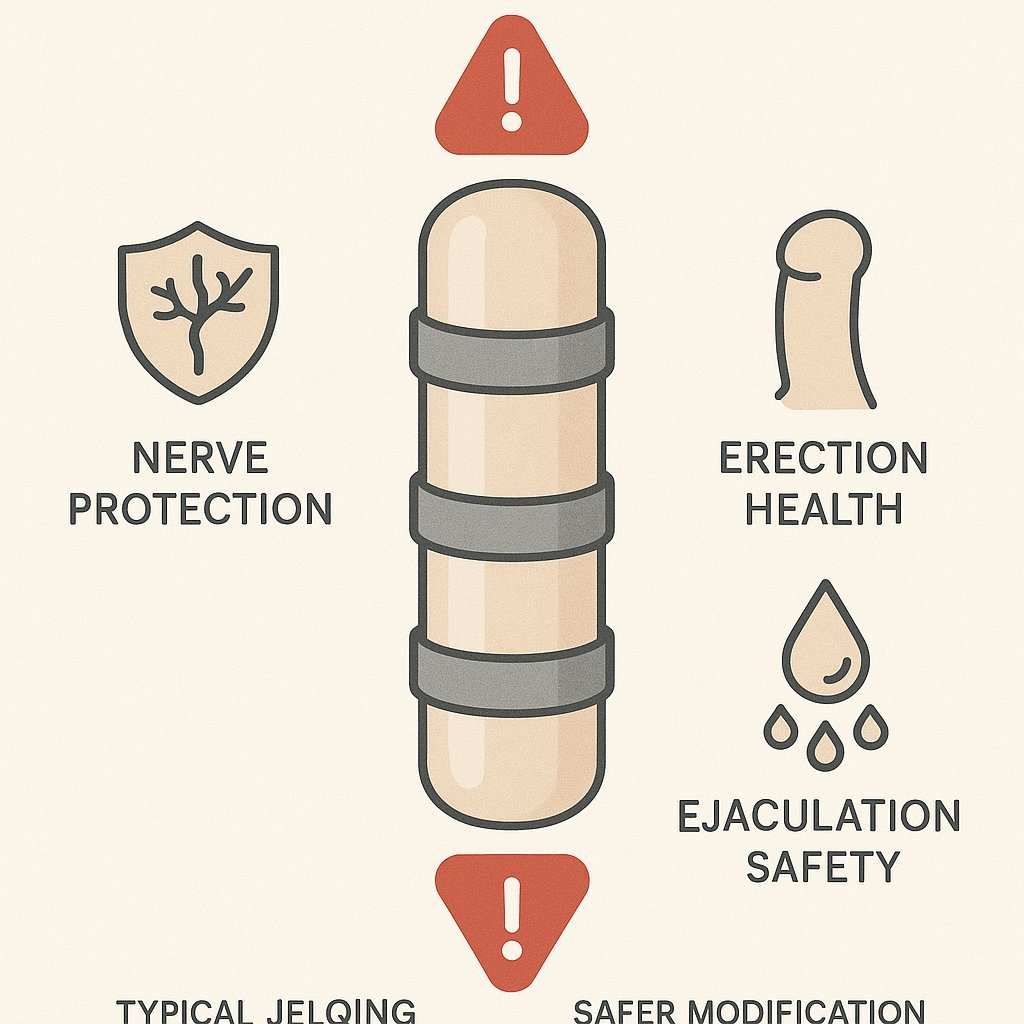
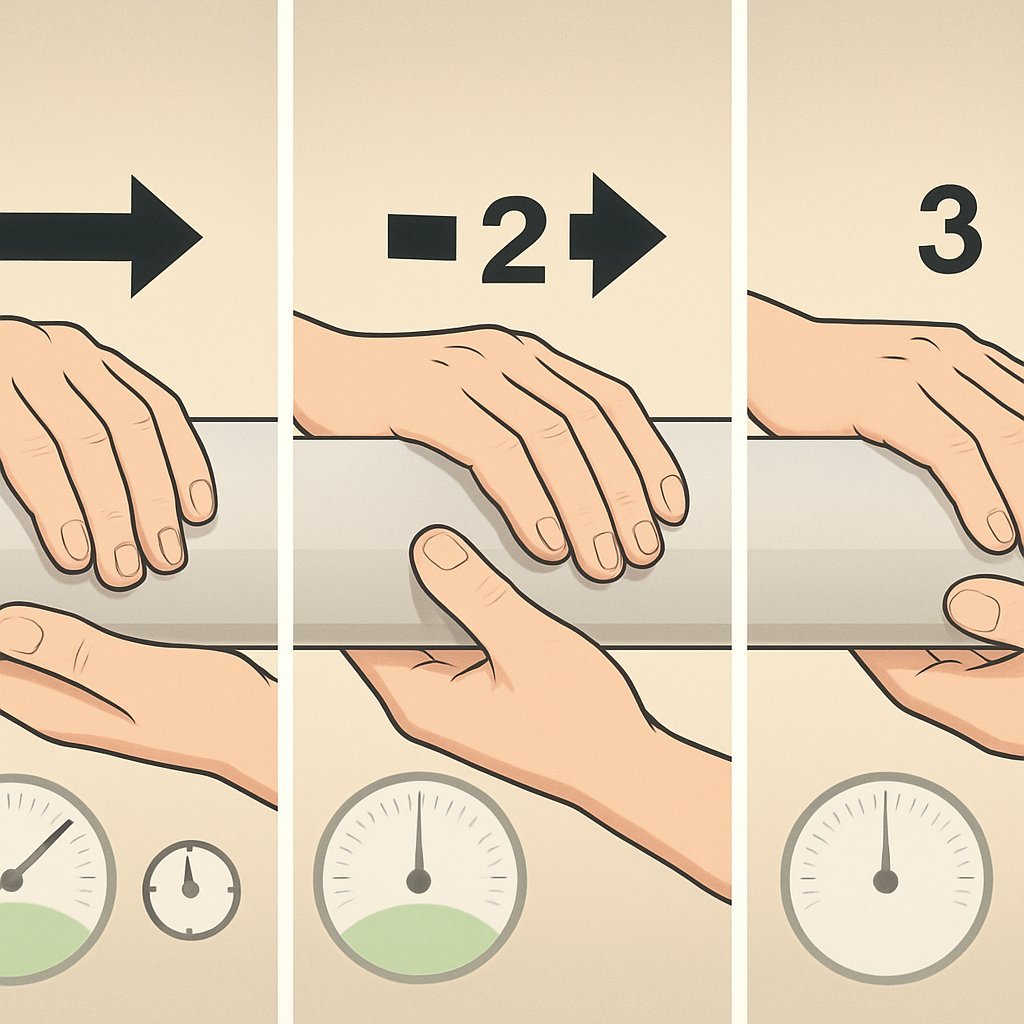
Jelq Basics for Beginners: Your Playful Guide to Getting Started
Are you ready to embark on a journey into the playful world of jelqing? Whether you stumbled here by accident or you’re seriously considering adding a new dimension to your self-care routine, you’ve come to the right place! But hold your horses—before we dive in, it’s important to note that while many have found this technique helpful, results can vary, and it’s always wise to approach any lifestyle change with consideration and a splash of fun!
Now that we’ve cleared that up, let’s get into the nitty-gritty of jelqing basics. This guide will not only introduce you to the art of jelqing but also help you navigate your way through the essential tools, techniques, and tips to get started confidently. We’ll cover everything you need to know, so grab a comfy seat and let’s explore this cheeky topic together!
## Understanding Jelqing: The Basics
Jelqing is often described as an exercise that aims to enhance your intimate self-care experience. Think of it as a playful way to explore your own body, like getting to know a new friend—except this friend is you! The movement is all about gripping and pulling—sounds simple, right? But it’s important to approach with a light heart and an open mind.
## Methodology: How We Chose Our Picks
To create this list of must-know items and techniques, we scoured the internet, consulted playful communities, and gathered insights from beginner-friendly forums. Our aim? To curate a list that’s not only informative but also engaging, ensuring that you can embark on your jelqing journey with confidence!
## The Playful Picks: What You Need to Know
Here are our top five playful picks that will set you on the path to jelq greatness:
Top Picks
Jelqing Guide for Beginners
This comprehensive guide takes you through the basics of jelqing, ensuring you have a solid foundation to start your journey.
Check priceJelq Better Manual
This manual features practical tips and techniques to make your jelqing experience enjoyable and effective.
Check priceThe Ultimate Jelq Stretch
An easy-to-follow stretch routine designed for beginners to accompany their jelqing journey.
Check priceComparison table
| # | Name | Rating | Price | |
|---|---|---|---|---|
| 1 | Jelqing Guide for Beginners | 4.5/5 | $19.99 | View |
| 2 | Jelq Better Manual | 4.2/5 | $14.99 | View |
| 3 | The Ultimate Jelq Stretch | 4.0/5 | $9.99 | View |
| 4 | Fun Jelq Journal | 4.8/5 | $12.99 | View |
| 5 | Jelqing Essentials Kit | 4.6/5 | $39.99 | View |
Full list
1. Jelqing Guide for Beginners
This comprehensive guide takes you through the basics of jelqing, ensuring you have a solid foundation to start your journey.
- Easy to follow
- Well-structured
- Supportive community
- May require patience
- Results vary per individual
2. Jelq Better Manual
This manual features practical tips and techniques to make your jelqing experience enjoyable and effective.
- Clear explanations
- Helpful visuals
- Encouraging tone
- Not a one-size-fits-all
- Requires consistency
3. The Ultimate Jelq Stretch
An easy-to-follow stretch routine designed for beginners to accompany their jelqing journey.
- Simple stretches
- Quick routine
- Balance and flexibility
- May take time to master
- Not a standalone solution
4. Fun Jelq Journal
This whimsical journal lets you track your progress and jot down your thoughts while having fun!
- Personalized
- Creative outlet
- Trackable progress
- May require commitment to fill out
- Not for everyone
5. Jelqing Essentials Kit
A complete kit designed for beginners, including everything you need to start jelqing safely.
- All-in-one kit
- Quality accessories
- Beginner-friendly
- Initial investment
- Limited to kit contents